As market demand for lithium-ion batteries continues to grow, problems such as lithium resource shortages and rising prices have gradually emerged.
Lithium and sodium, both alkali metals in Group IA of the periodic table, share similar physical and chemical properties, theoretically making them suitable as metal ion carriers in secondary batteries. Sodium-ion battery (SIB) share similar energy storage mechanisms as lithium-ion battery, boasting acceptable specific capacities and potentials. Therefore, SIBs are considered a viable alternative to lithium-ion batteries and have become a key focus of research and development.
Basic Structure of Sodium ion Battery
The structure of sodium-ion battery is essentially the same as that of lithium-ion battery, consisting primarily of positive electrode, negative electrode, electrolyte, separator, and current collector.
Based on whether the components of a sodium-ion battery directly participate in the electrochemical reaction, they can be divided into active and inactive materials. Active materials include the positive electrode material, the negative electrode material, and the electrolyte material. Inactive materials include the separator, the current collector, conductive agent, and binder.
The structure and properties of the positive and negative electrode materials in a sodium-ion battery determine the sodium storage performance of the entire battery. The electrolyte acts as a medium for ion flow between the positive and negative electrodes, allowing sodium ions to move between the electrodes while preventing the direct flow of electrons. The separator separates the positive and negative electrodes, preventing short circuits while allowing ion transport. The current collector collects and transmits electrons.
Sodium ion Battery Working Principle
Sodium-ion battery not only share a similar structure to lithium-ion battery, but also operate on essentially the same principles. They both work by ions being inserted and removed from the battery’s positive and negative electrodes.
The working principle of sodium-ion battery is that sodium ions move reversibly between the positive and negative electrodes through the electrolyte, accompanied by the flow of electrons through an external circuit. This process can be divided into two phases: charging and discharging.
Charging
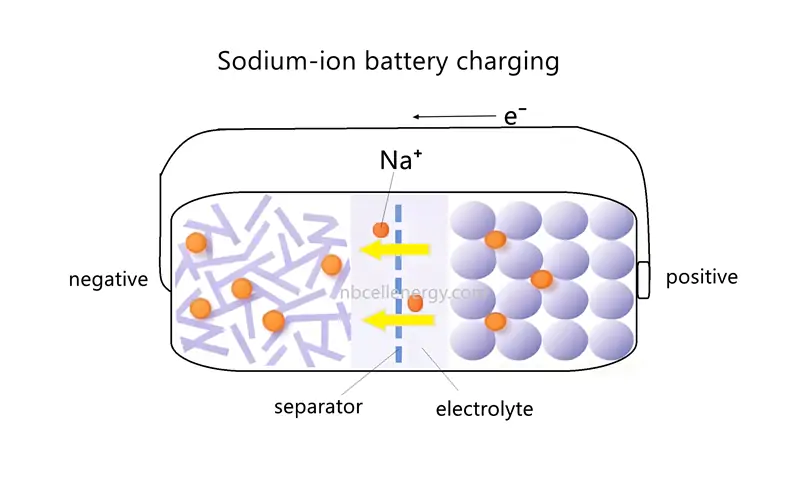
When sodium-ion battery is charged, the positive electrode acts as the anode (where an oxidation reaction occurs), and the negative electrode acts as the cathode (where a reduction reaction occurs).
During this process, an external power source applies a voltage, driving sodium ions (Na⁺) from the positive electrode through the electrolyte to the negative electrode. Electrons flow from the positive electrode to the negative electrode via an external circuit. The negative electrode material accepts sodium ions from the positive electrode and electrons from the external circuit. The sodium ions are embedded in the negative electrode’s structure, and electrons enter the negative electrode through the external circuit, maintaining charge balance.
Discharging
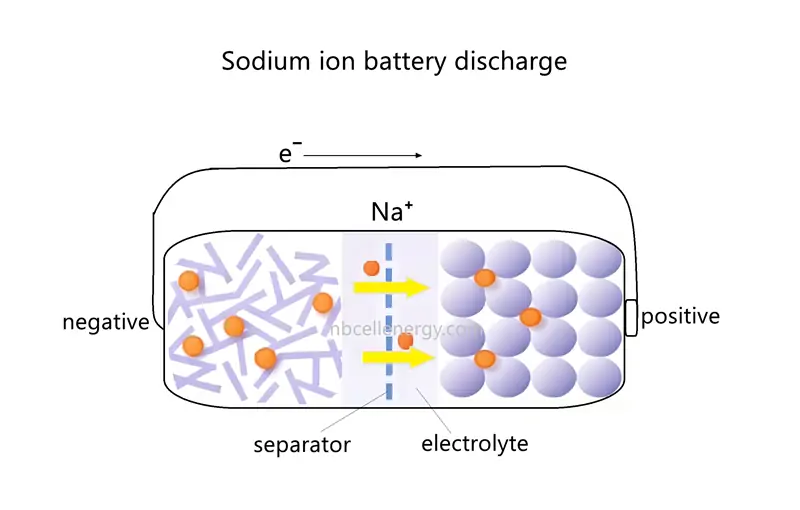
During discharge, the negative electrode of sodium-ion battery acts as the anode (where an oxidation reaction occurs), and the positive electrode acts as the cathode (where a reduction reaction occurs).
The discharge process of sodium-ion battery is the reverse of the charging process. Sodium atoms stored in the negative electrode material lose electrons, forming sodium ions (Na⁺). These sodium ions move from the negative electrode back to the positive electrode through the electrolyte. Electrons flow from the negative electrode to the positive electrode through an external circuit, powering connected devices. Sodium ions enter the positive electrode’s crystal structure through the electrolyte, while electrons enter the positive electrode through the external circuit, maintaining charge balance.
Electrochemical Process
The movement of sodium ions and electrons in sodium-ion batteries relies on several key electrochemical processes:
Intercalation/Deintercalation: In most sodium-ion batteries, both the cathode and anode materials are layered or porous, capable of reversibly storing sodium ions in their crystal lattices. For example, hard carbon anodes have a disordered structure that accommodates sodium ions, while layered oxide cathodes (such as NaNi₀.₅Mn₀.₅O₂) allow sodium ions to move in and out of the interlayer space.
Redox Reactions: Transition metals (such as Ni, Mn, and Fe) in the cathode undergo oxidation and reduction reactions to balance the intercalation and release of sodium ions. For example, in NaFePO₄, the Fe²⁺/Fe³⁺ redox couple drives the electrochemical reactions.
Ion Transport: The electrolyte must have high ionic conductivity to enable efficient sodium ion transport. Common electrolytes include sodium hexafluorophosphate (NaPF₆) dissolved in organic solvents such as ethylene carbonate (EC) and propylene carbonate (PC). Solid-state electrolytes, such as sodium β-alumina, are also being explored for improved safety.
Solid Electrolyte Interphase (SEI): During the first charge cycle, a thin SEI film forms on the anode surface due to electrolyte decomposition. This film passivates the anode, preventing further electrolyte degradation while allowing sodium ion transport. Due to the larger size of Na⁺ ions, the SEI film in solid electrolyte interphase (SIB) is less stable than in lithium-ion batteries, posing a challenge to cycle life.